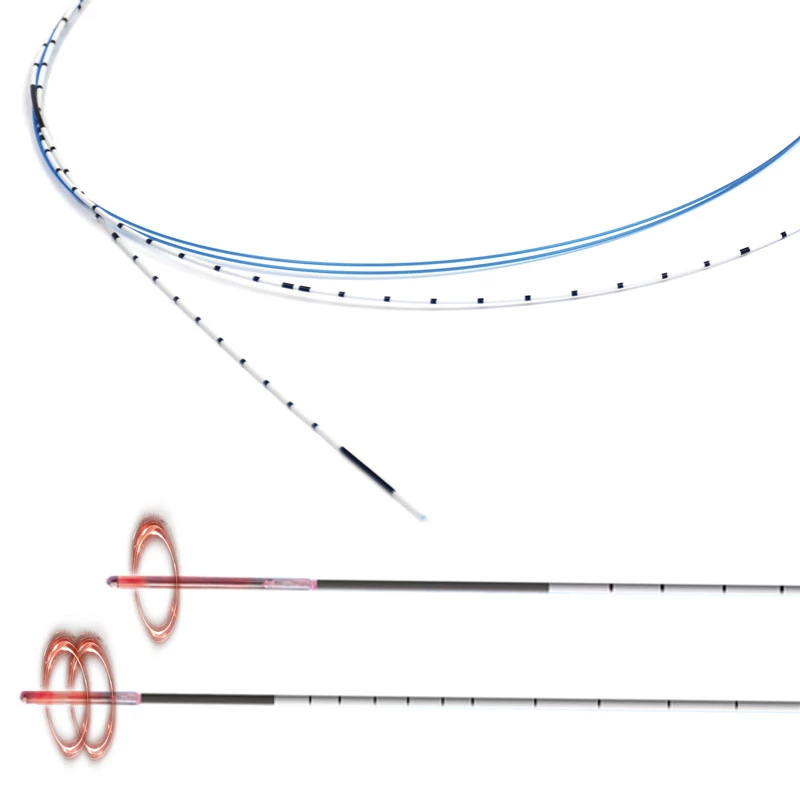
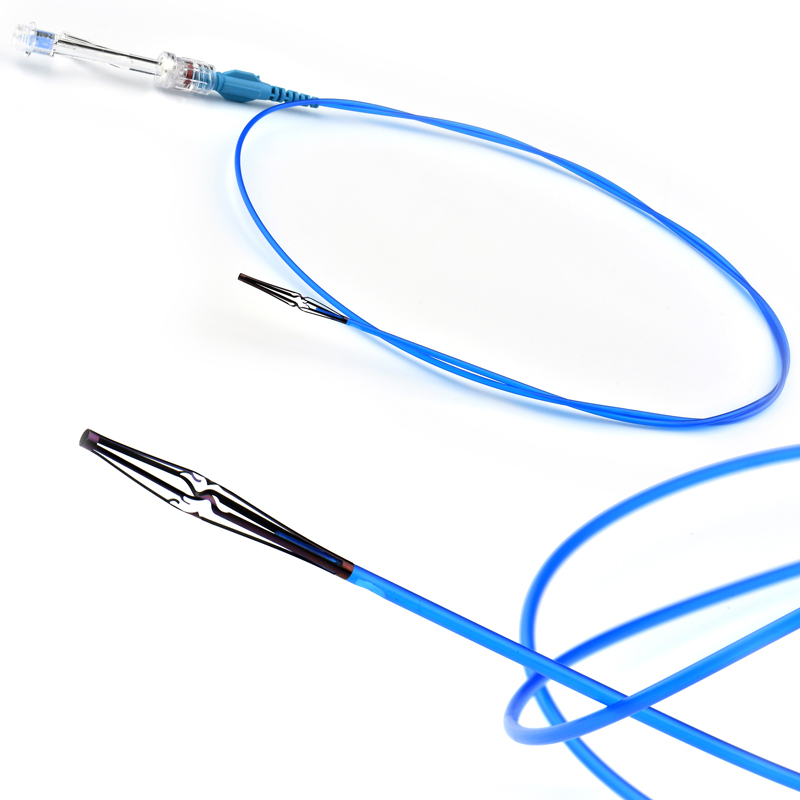
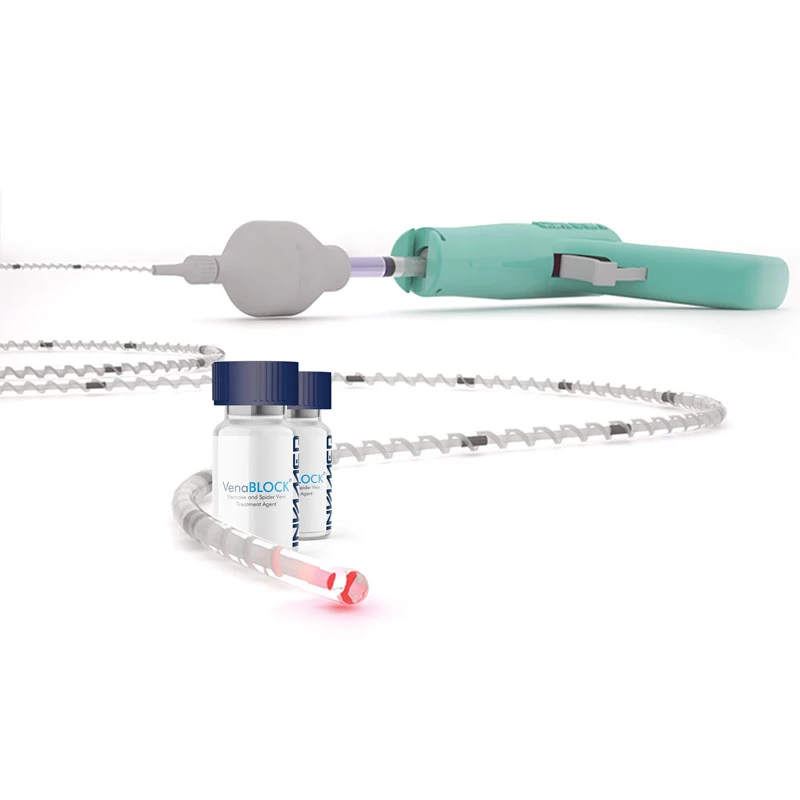
VenaBLOCK
Varicose Vein Closure System
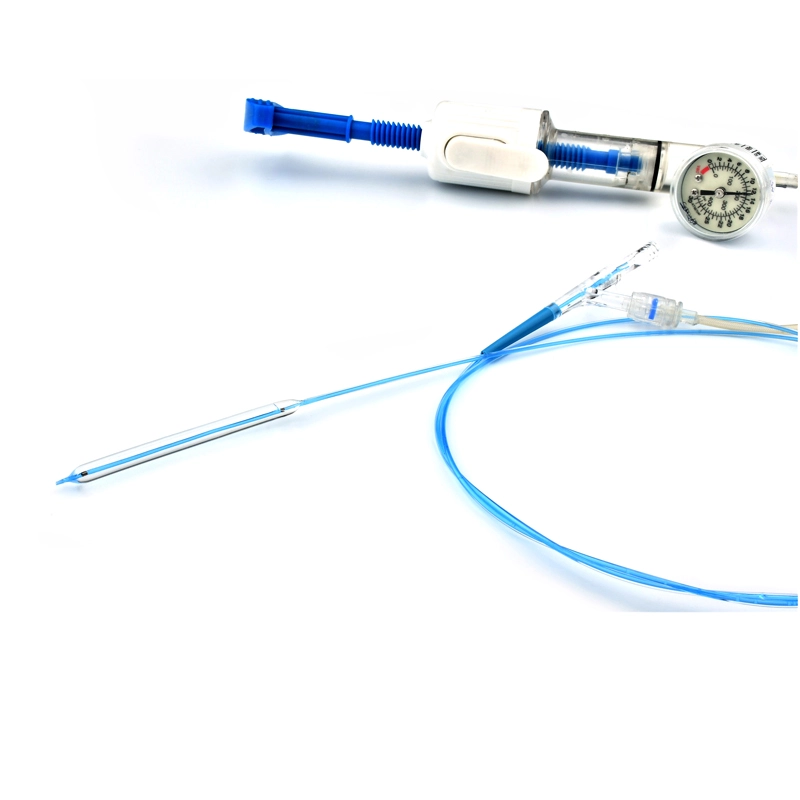
VenaBLOCKⓇ offers a unique treatment for venous insufficiency. Treatment is simply delivering cyanoacrylate inside of the dysfunctioning vein segment. (VSM, VSP, or perforator veins) continuously while applying pressure over target vein segments
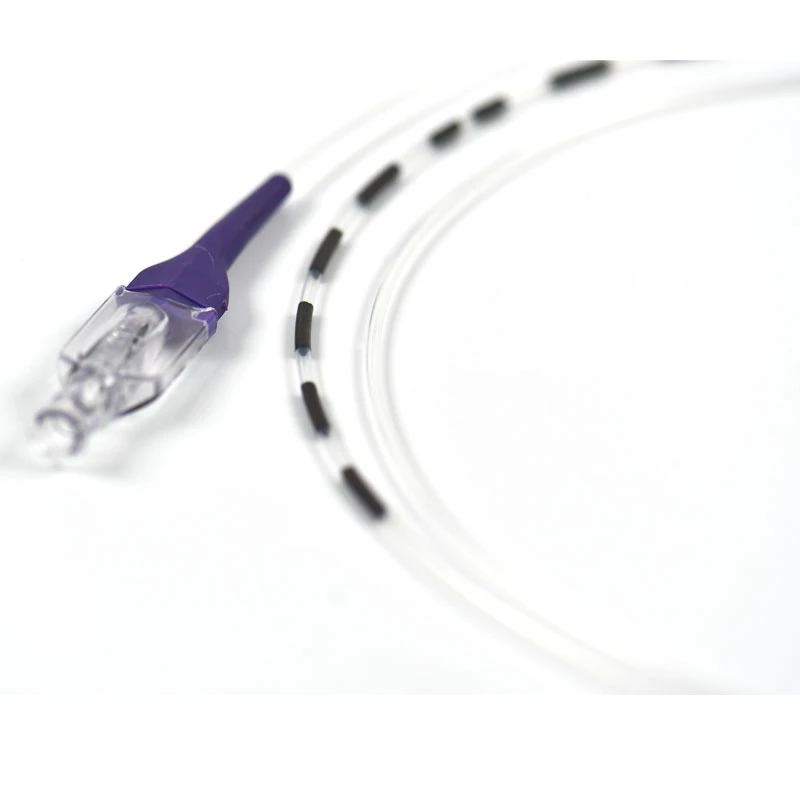
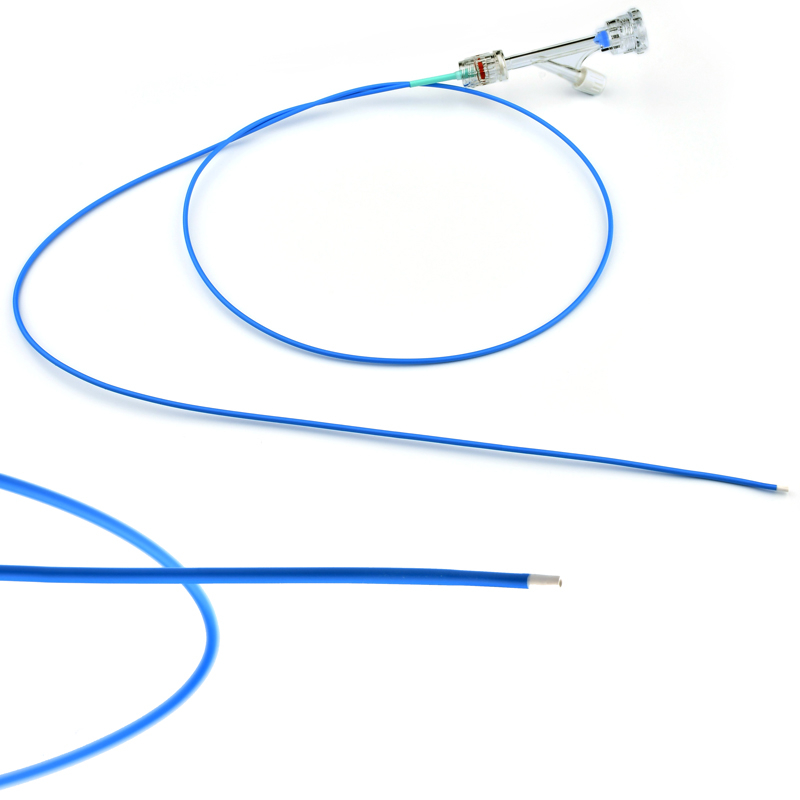
| Catheter Lenght | 100 cm PTFE |
|
| Catheter Material | Marked on every 2 cm OD: 2mm | |
| Marker | Double lumen; ID:0,5 mm for agent ID:0,9 mm for laser |
|
| Catheter Diameter | 15 cm | |
| Catheter Shaft | Braided, laser Guided |
VenaBlock Vein Sealing System 93/42/EEC is a Class III Medical-Surgery product that has the qualities required by “The EU Directive, (can be used in surgeries in and out of the body). VenaBlock is an n-BCA-polymer structure that was changed by adding a monomer synthesized by the producing company. It is ready for use, blue-colored, transparent liquid. Product show polymerization effect with embolization. When contacted with living tissue and in humid environments, it very fast becomes polymerized by forming a thin, flexible membrane with high tension resistance that ensures sticking firmly to the tissues. The subject matter membrane ensures a natural fit with the structure of the tissues, it is watertight and does not affect the structures of the blood and organic liquids. This formed membrane, since the polymerization of the product does not cause the occurrence of glassy components, can easily be perforated with a surgical needle after it is hardened. The polymerization time changes according to the type of tissue polymer contacted, characteristics of the liquid foundation, and the amount of the product used. When it is applied in the right conditions, VenaBlock starts polymerization after 1-2 seconds and finishes the polymerization process in about 5 seconds. In normal surgical operations, the membrane formed by it is eliminated from the body through the hydrolytic deterioration process; the time of the mentioned process depends on the type of tissue and amount of the polymer used. Whereas in the embolization processes, the polymer stays for a longer time. The polymerization reaction creates a temperature of about 45oC.





Detailed specifications
A UNIQUE TREATMENT FOR VENOUS INSUFFICIENCY CAN BE PERFORMED IN MINUTES
The physician only needs USG guidance to perform the procedure in outpatient conditions within several minutes. VenaBLOCKⓇ brings advantages such as eliminating tumescent anesthesia, ease of use, shortening the procedure time, and prevention of complications such as burn marks, bruising, hematoma, numbness, and pigmentation in thermal ablation methods.
The VenaBLOCKⓇ closure system delivers immediate and lasting vein closure with its proprietary medical adhesive formula, with a demonstrated 94.6% closure rate at 3 years.
Total Amounts of VenaBlock Embolization Liquid Expected for Use in Vein Treatments
The amount of adhesive delivered is related to the length of the target GSV. The VenaBLOCKⓇ Instructions for Use describe the application of 0.03 cc of the VenaBlock embolization liquid every cm in the target vein. The table below shows a calculation of the total expected amount of adhesive delivered as a function of the target treatment length. Additional injections could be administered at sites of dilatation and junctions with tributaries. The exact application procedure and dosage are to be determined by the physician.
SCIENTIFIC EVIDENCE
- %91.1 Closure Rate at 5 year - sciencedirect.com
- % 97.4 Closure Rate at 1 Year - [PDF]
- https://journals.sagepub.com/doi/abs/10.1177/02683555211025181
- https://www.sciencedirect.com/science/article/pii/S1051044318325260
- https://journals.sagepub.com/doi/abs/10.1177/17085381211013975
- https://dergipark.org.tr/en/download/article-file/1172156
- https://journals.sagepub.com/doi/abs/10.1177/17085381211051494
- https://www.jvascsurg.org/action/showPdf?pii=S0741-5214%2818%2931962-1
- https://link.springer.com/article/10.1007/s12262-020-02191-1
- https://grupologsa.com/qz-res/uploads/Venablock-Clinical-Study.pdf
- https://arya.mui.ac.ir/article_10811_900270b31f28fbfac9c56ad71f37633d.pdf
- https://journals.sagepub.com/doi/abs/10.1177/1708538118770548
- https://www.researchgate.net/profile/Imre-Bihari/publication/347731328
(FOR REFERENCE ONLY) Total amount of adhesive delivered as a function of planned treatment length
|
Planned Treatment Length (cm) |
Total Amount of NBCA (cc) |
|
5 |
0,15 |
|
10 |
0,3 |
|
15 |
0,45 |
|
20 |
0,6 |
|
25 |
0,75 |
|
30 |
0,9 |
|
35 |
1,05 |
|
40 |
1,2 |
|
45 |
1,35 |
|
50 |
1,5 |
|
55 |
1,65 |
|
60 |
1,8 |
|
65 |
1,95 |
|
70 |
2,1 |
|
75 |
2,25 |
|
80 |
2,4 |
|
85 |
2,55 |
|
90 |
2,7 |
|
95 |
2,85 |
|
100 |
3 |
FEATURES
- Can be performed in outpatient conditions.
- Non-tumescent procedure. Short procedure time.
- Closure of the target vessel can be obtained in seconds.
- No burn marks, skin pigmentation or lesions, numbness.
- No need to use compression stocking after the procedure.
- Patients can return to daily routines and activities immediately.
- VenaBLOCKⓇ MicroCatheter
- Braided PTFE Catheter
- Laser-guided
- High echogenicity and better visibility under USG
- Excellent pushability
- Marked on every 2 cm
The VenaBLOCKⓇ closure system is indicated for use in the closure of saphenous veins (vena saphena Magna, valve saphenous Parva) or perforator veins in endovenous treatment of incompetent varicose veins. VenaBLOCKⓇis an n-BCA-polymer structure that was changed by adding a monomer synthesized by the producing company. It is ready for use, blue-colored, transparent liquid. Product show polymerization effect with embolization. When contacted with living tissue and in humid environments, it very fast becomes polymerized by forming a thin, flexible membrane with high tension resistance that ensures sticking firmly to the tissues. The subject matter membrane ensures a natural fit with the structure of the tissues, it is watertight and does not affect the structures of the blood and organic liquids. This formed membrane, since the polymerization of the product does not cause the occurrence of glassy components, can easily be perforated with the surgical needle after it is hardened. The polymerization time changes according to the type of tissue polymer contacted, characteristics of the liquid foundation, and the amount of the product used. When it is applied in the right conditions, VenaBLOCKⓇ starts polymerization after 1-2 seconds and finishes the polymerization process in about 5 seconds.
POTENTIAL ADVERSE EFFECTS
Below is a list of the potential adverse effects (e.g., complications) associated with the use of theVenaBLOCKⓇ system. The adverse events associated with the device are similar to those with traditional endovenous thermal ablation procedures.
- Allergic reactions to cyanoacrylates, such as hives, asthma, hay fever, and anaphylactic shock
- Arteriovenous fistula
- Bleeding from the site of access
- Deep vein thrombosis (DVT)
- Edema in the treated leg
- Embolization, including pulmonary embolism (PE)
- Hematoma
- Hyperpigmentation
- Infection at the access site
- Non-specific mild inflammation of the cutaneous and subcutaneous tissue
- Pain
- Paresthesia
- Phlebitis
- Superficial thrombophlebitis
- Urticaria or ulceration may occur at the site of injection
- Vascular rupture and perforation
- Visible scarring
| Order Number | Catheter lenght | Catheter material | Marker | Catheter Outer Diameter | Catheter Inner Diameter | Catheter Shaft |
| VS1011 | 90 cm | PTFE | every 2 cm | 2 mm | Double lumen; ID:0,5 mm for agent ID: 0,9 mm for laser |
Braided, Laser Guided |
| VS1012 | 100 cm | PTFE | every 2 cm | 2 mm | Double lumen; ID:0,5 mm for agent ID: 0,9 mm for laser |
Braided, Laser Guided |