Atlas
Endovascular Stent Graft System
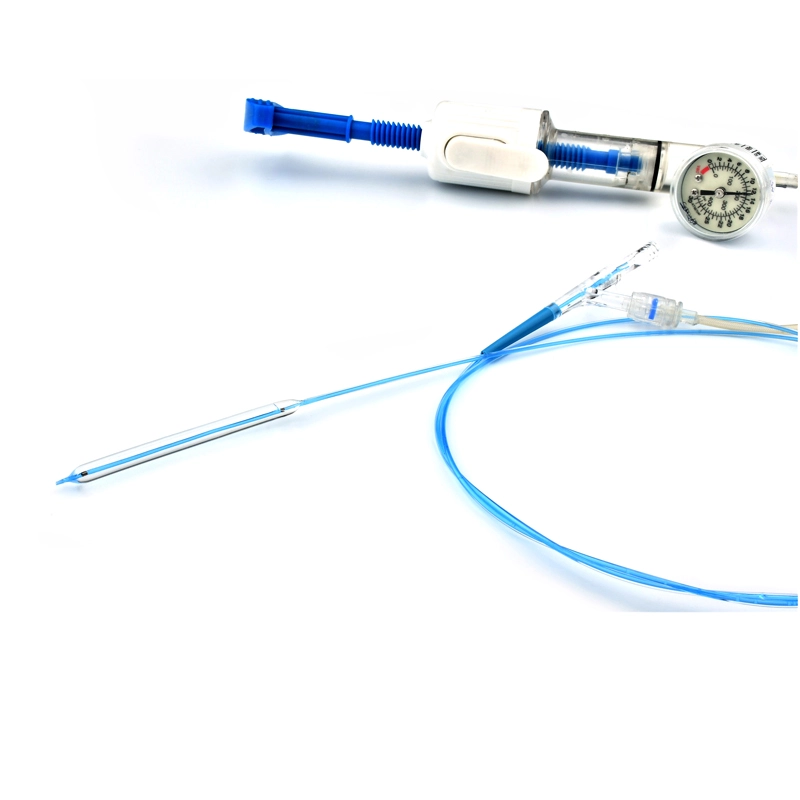
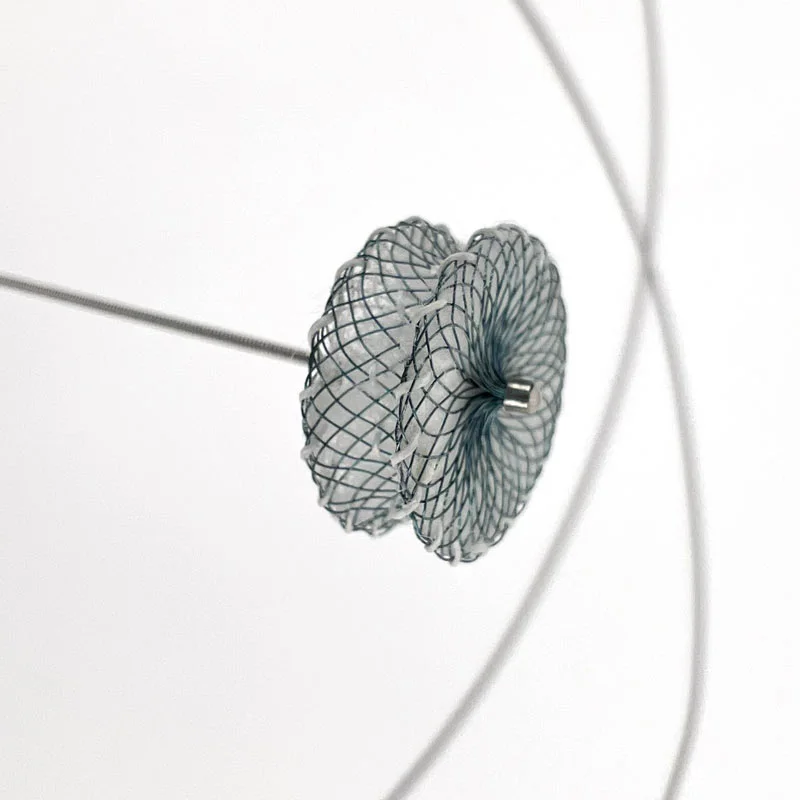
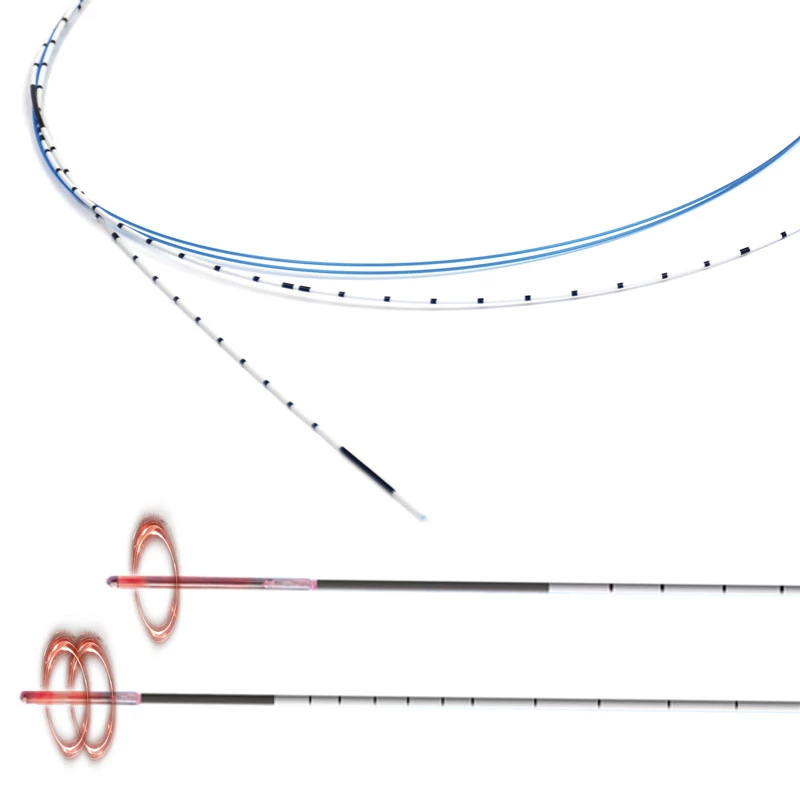
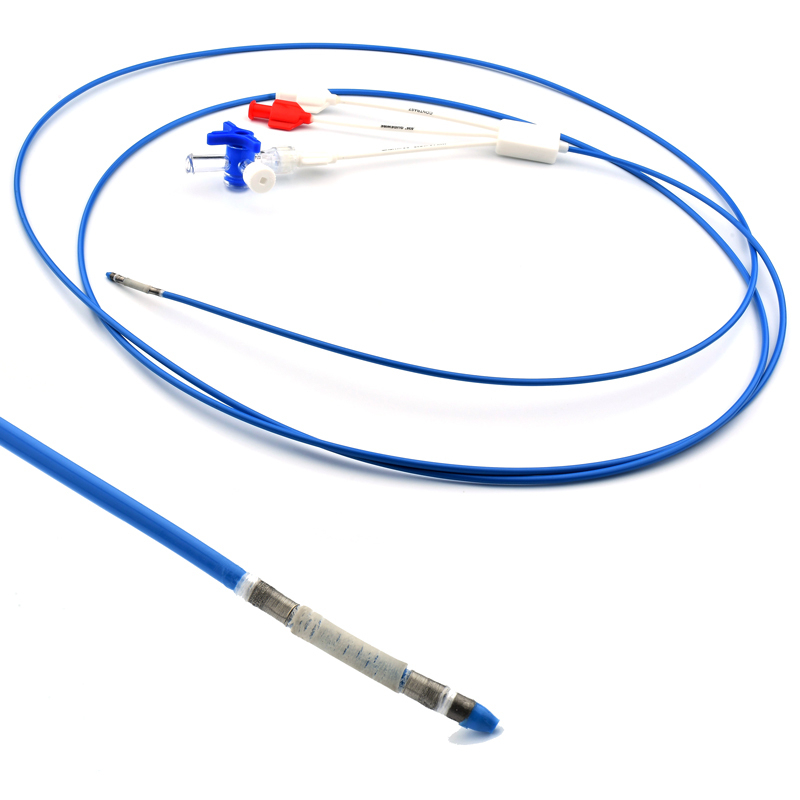
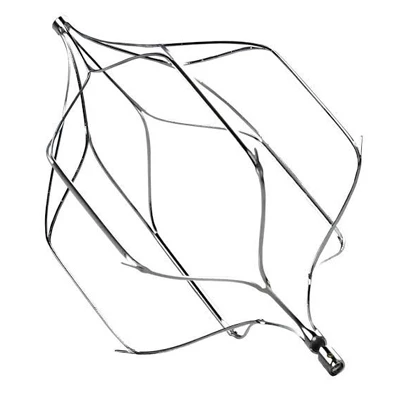
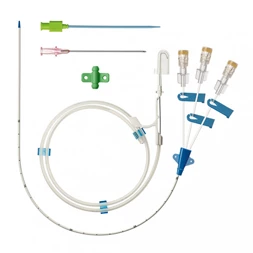
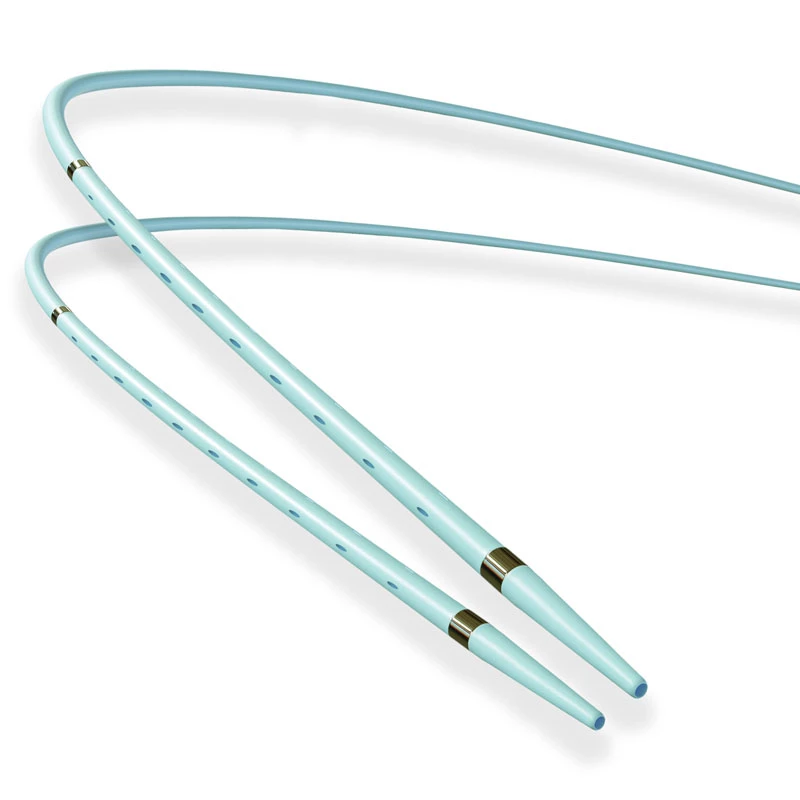
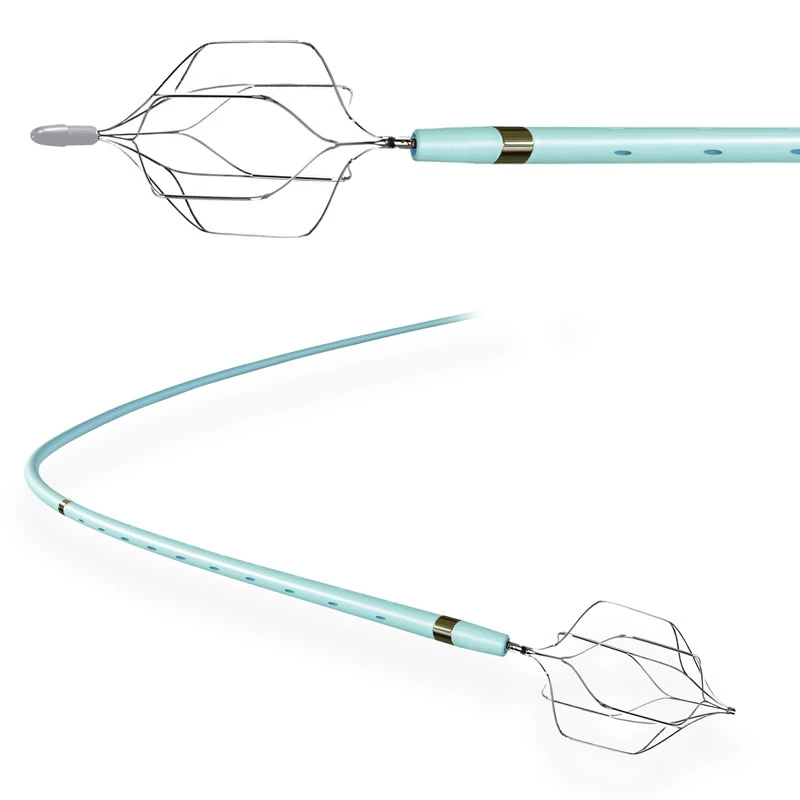
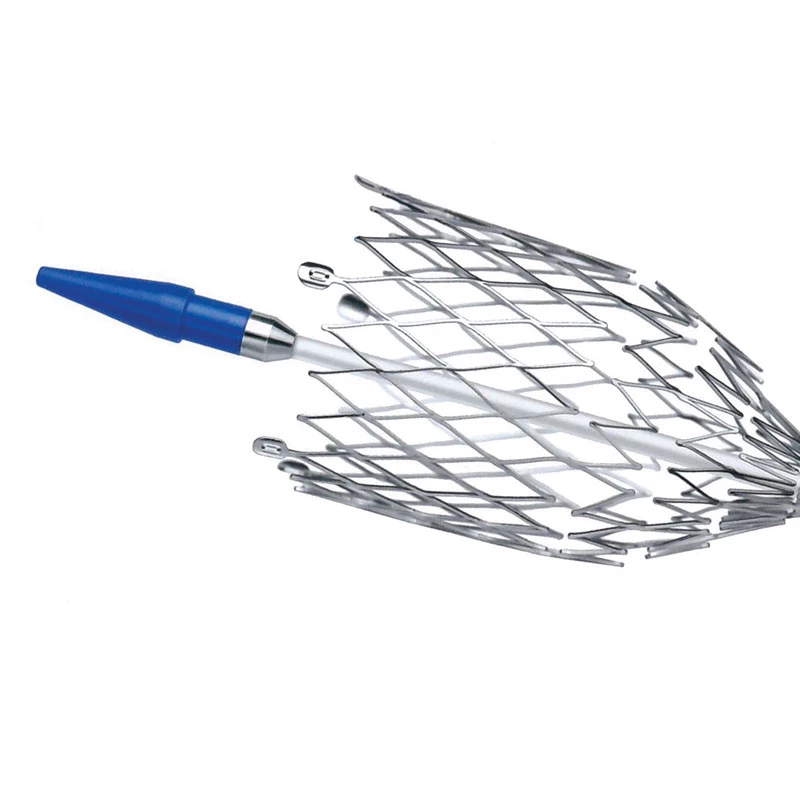
The AtlasⓇ endovascular stent graft (implant) is a self-expanding Nitinol stent covered with expanded Polytetrafluorethylene (EPTFE). Nitinol stent has markers on both ends with tantalum markings showing high radioopacity. Its surface is covered with carbon Various diameters and lengths are available.
| Graft Material | Microporous EPTFE | |
| Stent Material | Nitinol | |
| Stent Graft Design | Singel Stent | |
| Handling System | 6F & 7F | |
| Introducer Sheath Compatibility |
6F & 7F | |
| Guide Wire Compatibility | 0.035" | |
| Body Size | 120 cm | |
| Marker Mateial | Pt-lr | |
| Catheter Body Length | 120 cm | |
| Open Stent Graft Size | 5.0, 6.0, 7.0, 8.0, 9.0, 10.0 | |
| Nominal Stent Graft Length | 18, 22, 28, 38, 58 18, 23, 27, 37, 57 |
|
| Shelf Life | 36 Month | |
| Inner Lumen Surface | ePTFE Coated |
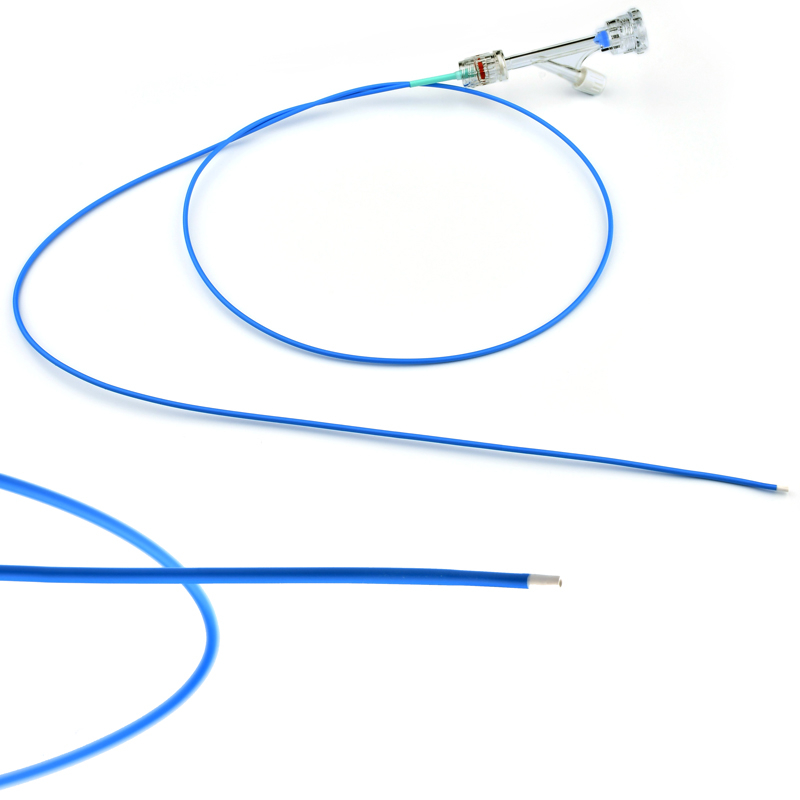
Atlas Endovascular Stent Graft System is a self-expanding Nitinol stent lined with expanded Polytetrafluoroethylene (ePTFE). Nitinol stent has a total of four radiopaque Tantalum Markers at both ends increase visibility under fluoroscopy and facilitate stent-graft insertion. The nitinol stent is lined with ePTFE along its entire length except at the two ends with radioactive tantalum markings. The luminal surface of ePTFE is coated with Carbon. Various diameters and lengths are available. The Stent Graft is delivered pre-seated between the inner catheter and the outer sheath, located in the distal portion of the delivery system.








Detailed specifications
AtlasⓇ Endovascular ePTFE Coated Stent Graft System
AtlasⓇ endovascular stent graft (implant) is a self-expanding Nitinol stent covered with expanded Polytetrafluorethylene (EPTFE).
Nitinol stent has markers on both ends with tantalum markings showing high radioopacity. Its surface is covered with carbon.
It is indicated for the treatment of upper extremity venous outflow stenosis of patients dialyzed with an arteriovenous (AV) access graft or fistula.
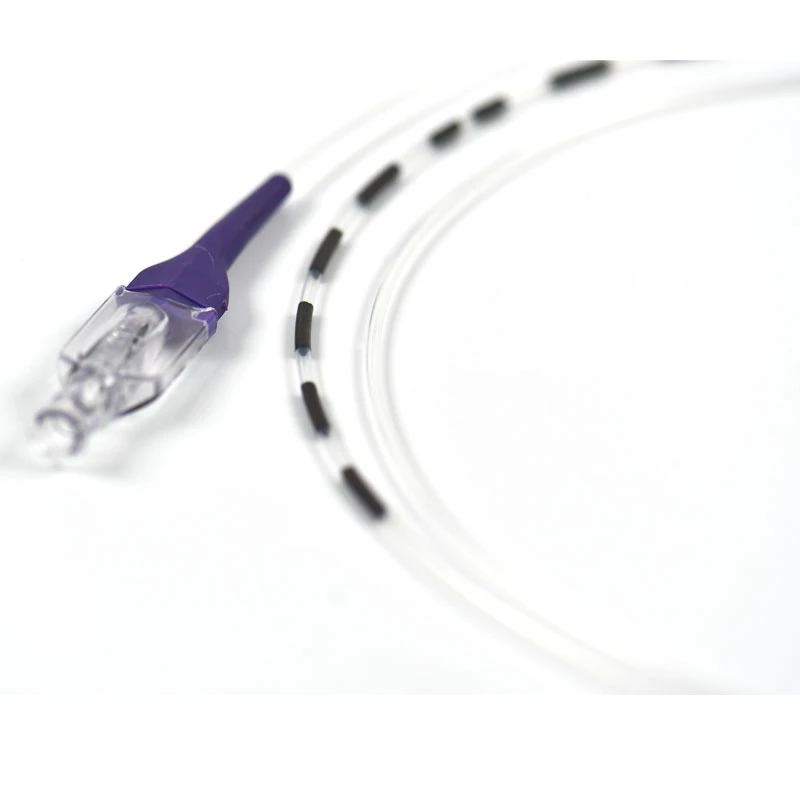
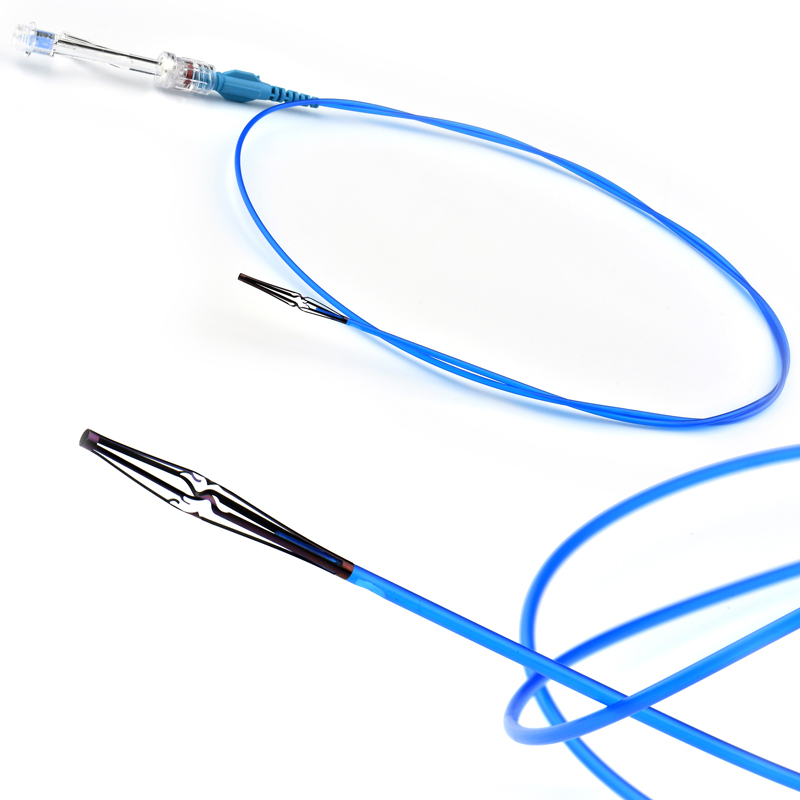
The stent is delivered inserted between the inner catheter and the outer sheath, located in the distal portion of the graft delivery system. In such a compressed arrangement, the supports of the nitinol stent are positioned close to each other.
The flexible release system is a coaxial catheter system consisting of an internal catheter integrated into the system via a metal tubing shaft and a coaxial outer sheath connected to the Y-injection adapter via the Tuohy-Borst valve.
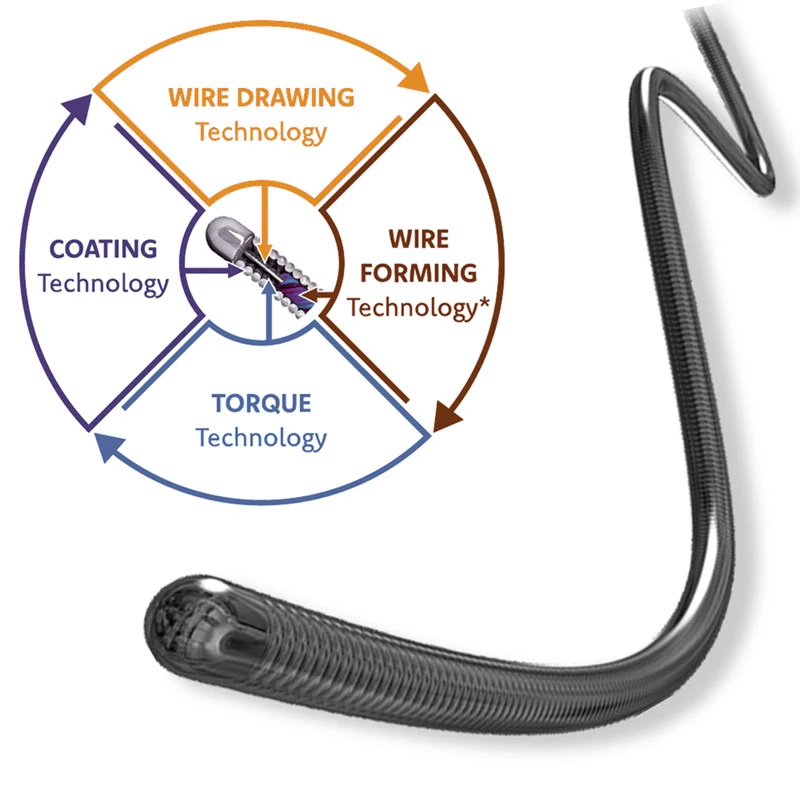
Superior Performance with Innovative Design.
Special Design for Radial Strength and Flexibility.
- Atraumatic tip designed to facilitate smooth entry and exit at the access point
- Straight and flared configurations to optimize hemodynamic flow in the venous anastomosis
- Full encapsulation between two ePTFE designed to resist neointimal hyperplasia at the treatment site
- Designed to withstand bending, compression, and stretching in combination with spiral posts and angled bridges
- Tantalum markers to increase visibility under fluoroscopy.
AtlasⓇ endovascular stent graft (implant) is a self-expanding Nitinol stent covered with expanded Polytetrafluorethylene (EPTFE).
Nitinol stent has markers on both ends with tantalum markings showing high radioopacity. Its surface is covered with carbon.
ePTFE Coated LUMINAL SURFACE MADE BY PLASMA ION IMPLANTATION METHOD
Grafts created with this technology both improve mechanical properties and increase radial strength. It provides high biocompatibility and reduces ion release and thrombogenicity.
Proven to have effective propulsion, tracking, and visibility under fluoroscopy on a low-profile delivery system in a preclinical model.
• ePTFE Coated ePTFE along the lumen surface to reduce early platelet adhesion
• Provides superior fluoroscopic imaging of the entire Stent Graft placement process with Tantalum Markers
• Excellent radial expansion strength minimizes the risk of Stent Graft dislocation or displacement
• Minimal stent graft that appears small during placement ensures placement accuracy
• AtlasⓇ Endovascular Stent Grafts are MRI conditional
AtlasⓇ Endovascular Stent Graft represents the perfect balance of desirable properties such as radial strength, flexibility, minimal shortening, low profile, placement accuracy, and convenient placement in an easy-to-use withdrawal delivery system.
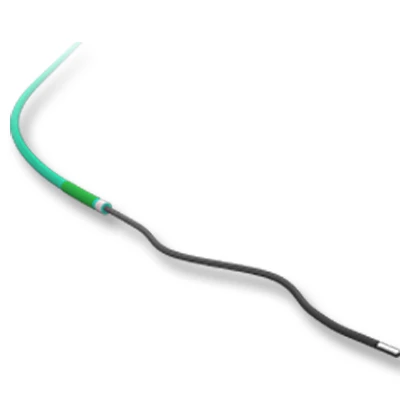
The delivery system is a multifunctional knitted catheter technology with an optimal balance between shaft Pushability and sustained Flexibility at the catheter tip, providing excellent Traceability in the target lesion area.
• Soft automatic tip is a conical tip suitable for 0.035” guide wire.
• “Topless internal catheter” reduces the risk of insertion after catheter entanglement Low profile delivery systems reduce patient trauma.
• Reduction of early platelet adhesion ePTFE with Carbon along the lumen surface is covered.
• Entire Stent Graft with Tantalum Markers superior fluoroscopic provides viewing.
• Minimal that appears small during placement stent-graft ensures placement accuracy
• AtlasⓇ Endovascular Stent Grafts MRI is conditional.
AtlasⓇ Endovascular Stent Graft radial strength, flexibility, minimal shortening low profile, placement accuracy, and in an easy-to-use pull-back transmission system proper placement
Superior Performance with Innovative Special Special Design for Radial Strength and Flexibility
• Smooth entry and exit at the access point atraumatic tip designed to facilitate
• The hemodynamic flow in the venous anastomosis flat and extended configurations
• Between two ePTFE, in the treatment area to resist neointimal hyperplasia designed full-encapsulation
• With spiral posts and angled bridges against bending, compression, and stretching designed to provide durability
• To increase visibility under fluoroscopy tantalum markers
| Graft Material | Microporous EPTFE | |
| Stent Material | Nitinol | |
| Stent Graft Design | Singel Stent | |
| Handling System | 6F & 7F | |
| Introducer Sheath Compatibility |
6F & 7F | |
| Guide Wire Compatibility | 0.035" | |
| Body Size | 120 cm | |
| Marker Mateial | Pt-lr | |
| Catheter Body Length | 120 cm | |
| Open Stent Graft Size | 5.0, 6.0, 7.0, 8.0, 9.0, 10.0 | |
| Nominal Stent Graft Length | 18, 22, 28, 38, 58 18, 23, 27, 37, 57 |
|
| Shelf Life | 36 Month | |
| Inner Lumen Surface | ePTFE Coated |
| CATHETER LENGTH (120 cm) | |||||||||
| Diameter | 18 mm | 22 mm | 23 mm | 27 mm | 28 mm | 37 mm | 38 mm | 57 mm | 58 mm |
| (mm) | |||||||||
| 5.0 | VS5018 | VS5022 | VS5023 | VS5027 | VS5028 | VS5037 | VS5038 | VS5057 | VS5058 |
| 6.0 | VS6018 | VS6022 | VS6023 | VS6027 | VS6028 | VS6037 | VS6038 | VS6057 | VS6058 |
| 7.0 | VS7018 | VS7022 | VS7023 | VS7027 | VS7028 | VS7037 | VS7038 | VS7057 | VS7058 |
| 8.0 | VS8018 | VS8022 | VS8023 | VS8027 | VS8028 | VS8037 | VS8038 | VS8057 | VS8058 |
| 9.0 | VS9018 | VS9022 | VS9023 | VS9027 | VS9028 | VS9037 | VS9038 | VS9057 | VS9058 |
| 10.0 | VS1018 | VS1022 | VS1023 | VS1027 | VS1028 | VS1037 | VS1038 | VS1057 | VS1058 |