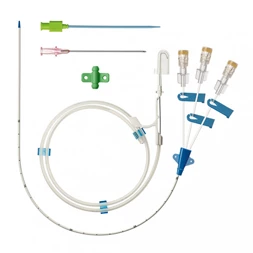
MicroCATH
Peripheral Catheter
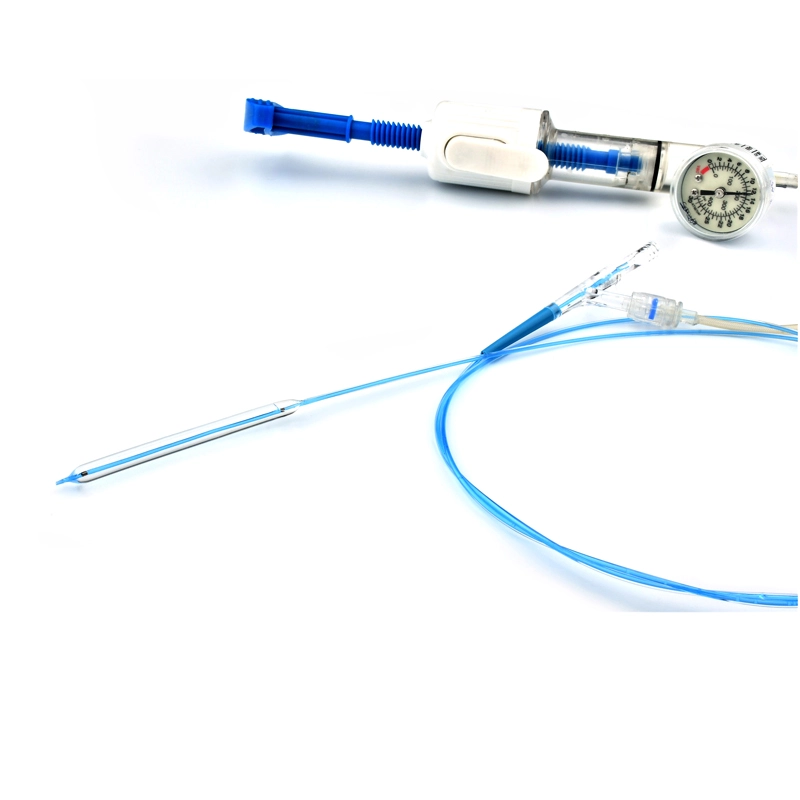
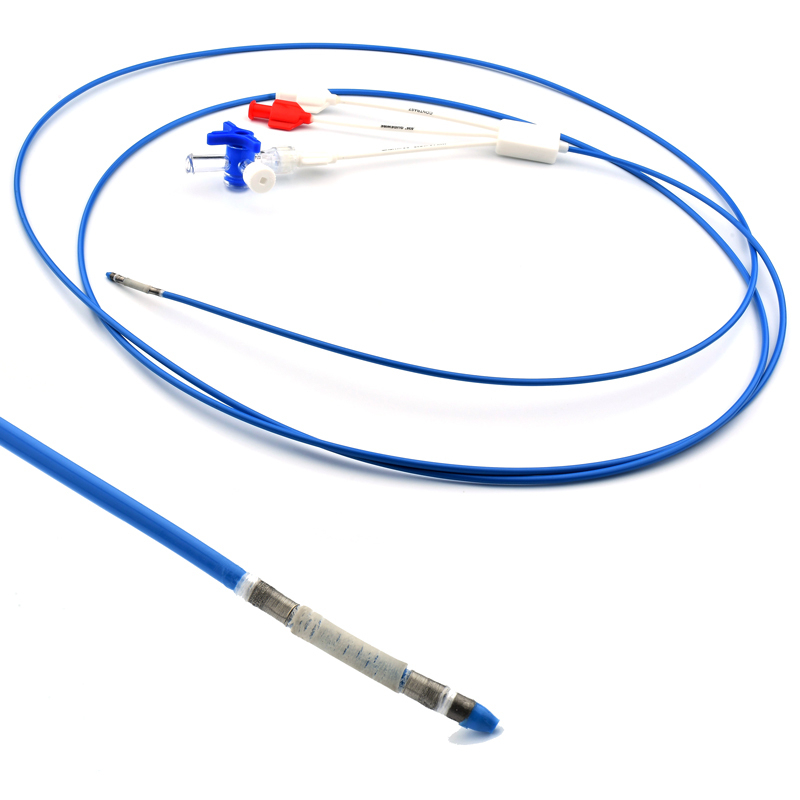
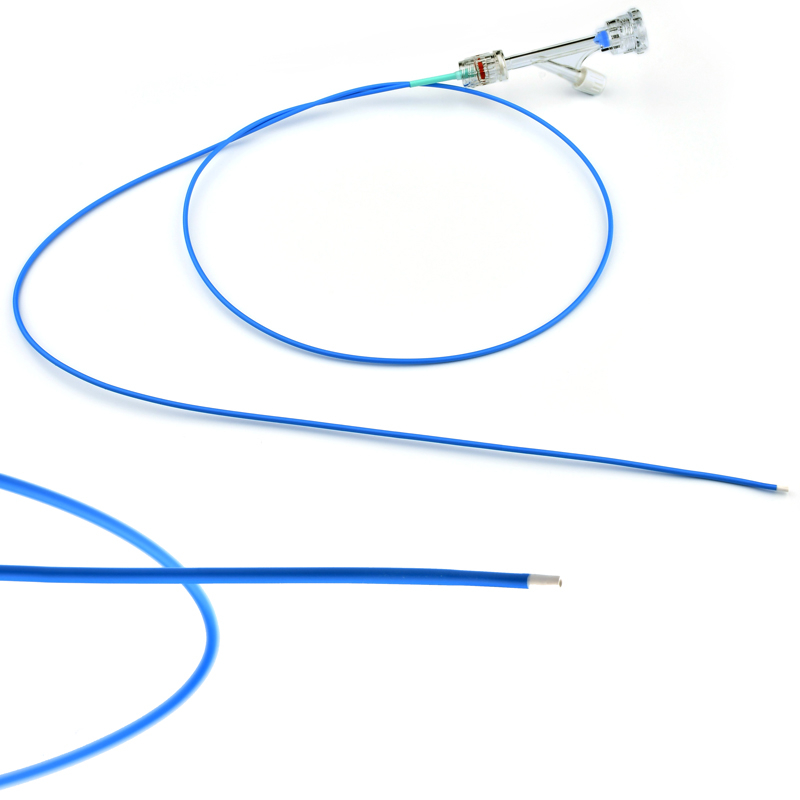
This MicroCATHⓇ Peripheral catheter is designed for infusion of contrast medium into peripheral vessels. The catheter is also designed for drug infusion in intra-arterial therapy and infusion of embolic materials for hemostasis.
A Safe Embolotherapy
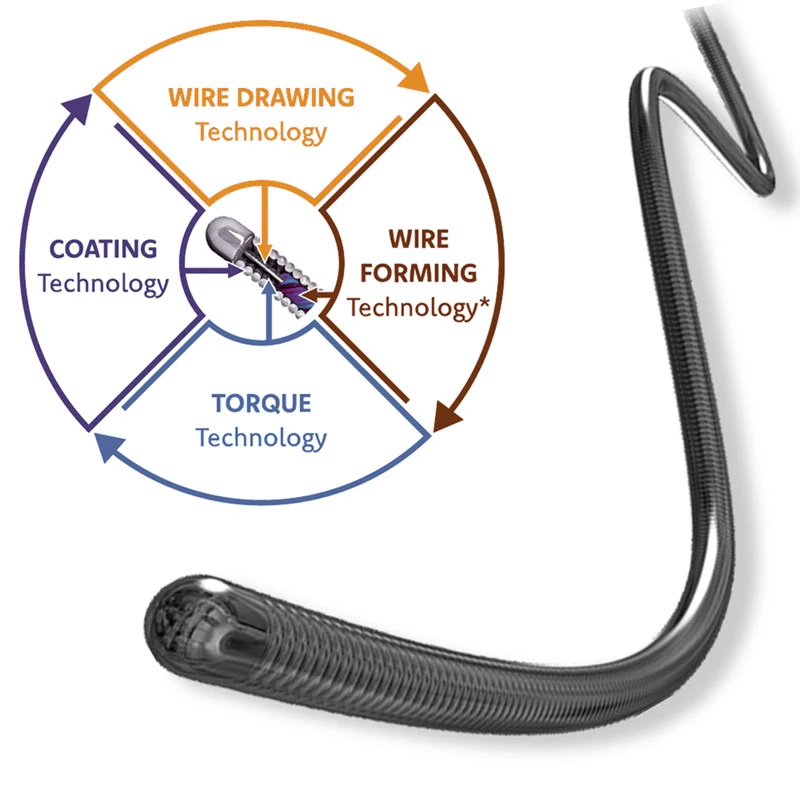
- Coil design reinforcement for bending resistance.
- PTFE inner layer designed for exceptional guidewire tracking with virtually frictionless conduction of coils and other embolics.
- DMSO Compatible.
- Based on mechanical testing.
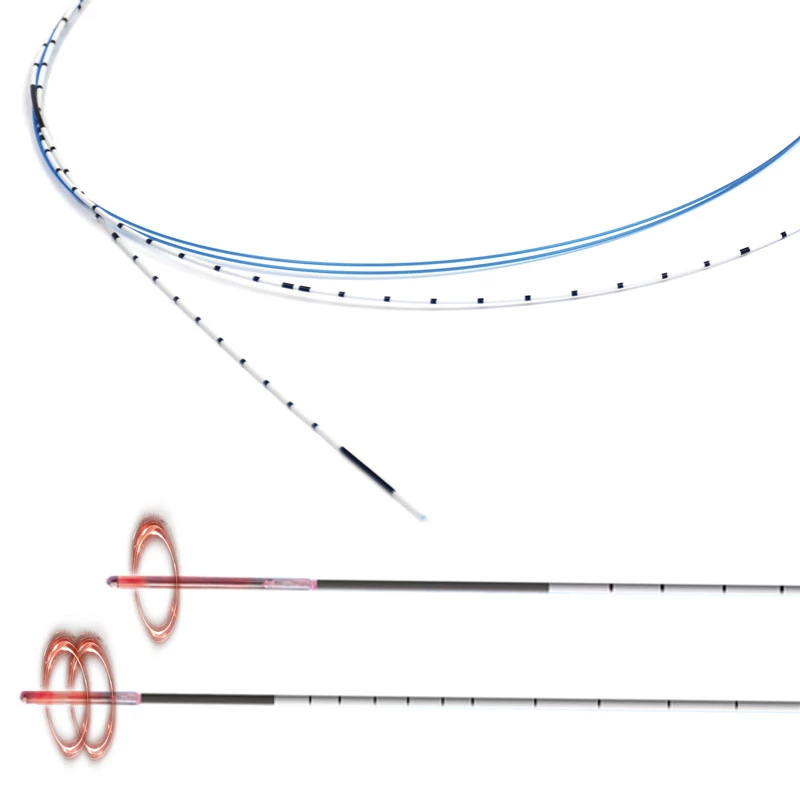
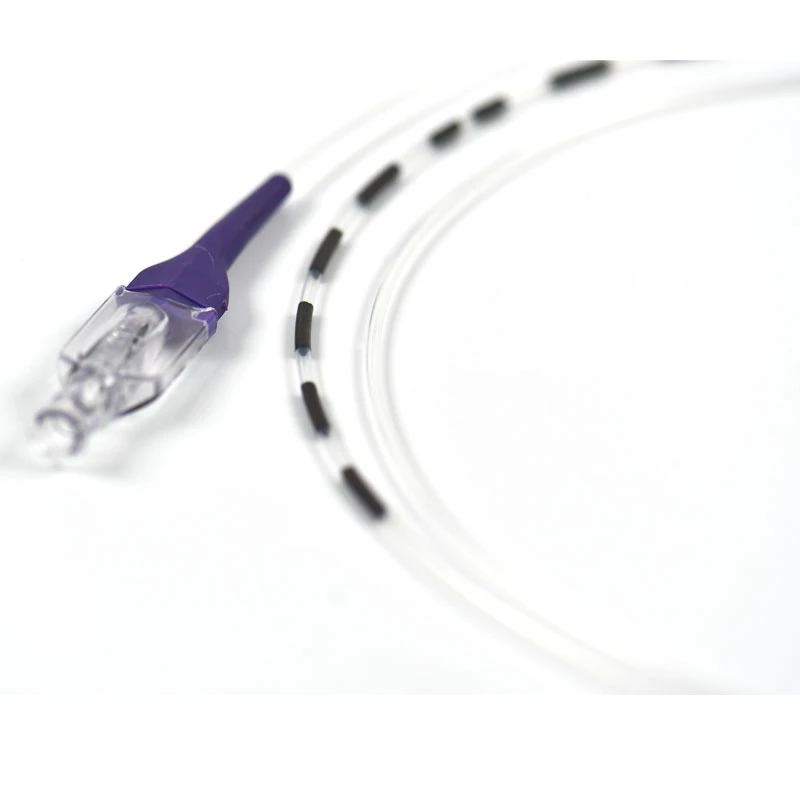
Invamed MicroCath Catheter products are Medical-Surgical products (which can be used inside the body in surgeries) with the qualifications required by MEDDEV 2.7.1 and the “EU Directive” numbered 93/42/EEC. There are different types according to different usage areas and diameters. It is suitable for the use of 0.014” micro guide wire and is supplied together. The tip has a radiopaque marker and is highly visible under ultrasound. 5F, 6F, and 7F diameter; It has 90 cm and 150 cm lengths and is compatible with embolizing agents. DMSO compatible. It has an internal diameter of 0.028 in (0.7 mm/0.6 mm/0.5 mm) flowing minimally in the distal regions. The microcatheter lumen can be contained within steerable guidewires 0.018 in (0.47 mm) in diameter or less. Embolizing agent is compatible.


Detailed specifications
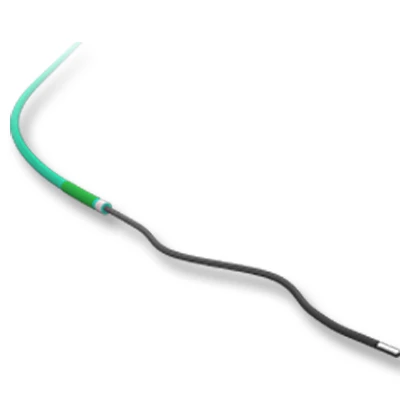
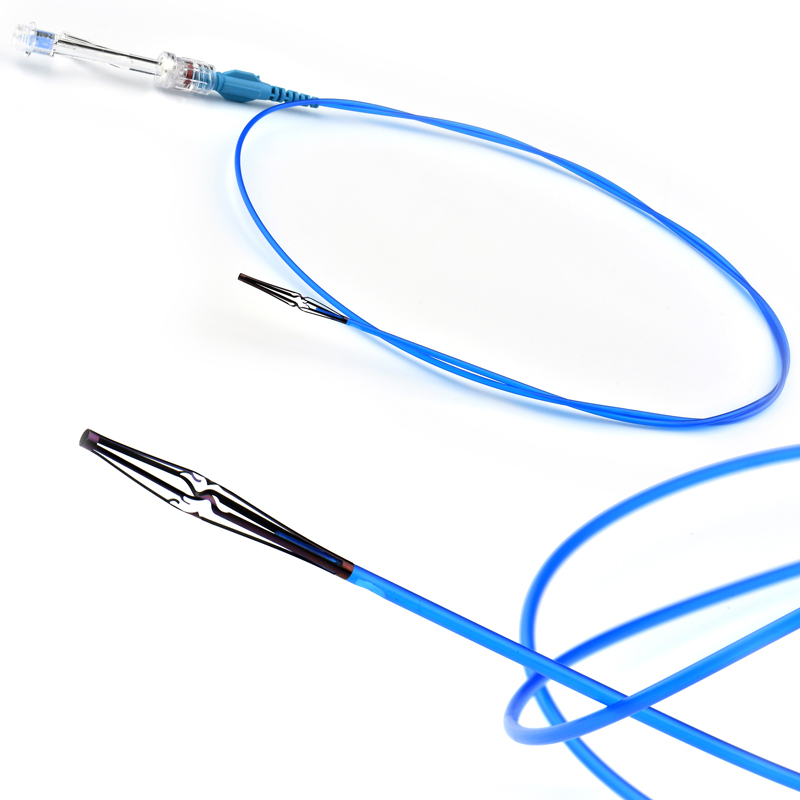
The MicroCATHⓇis designed to allow navigation in convoluted peripheral vessels for optimal access and delivery of therapeutic embolization. With the hydrophilic coating, you can reposition the microcatheter tip without a guidewire and achieve clear imaging with power injections up to 1200 psi, with the highest torque response and high-pressure flow rates in its class.
The catheter flexibility profile is designed with a soft distal tip to aid traceability and a harder proximal tip to aid pushability
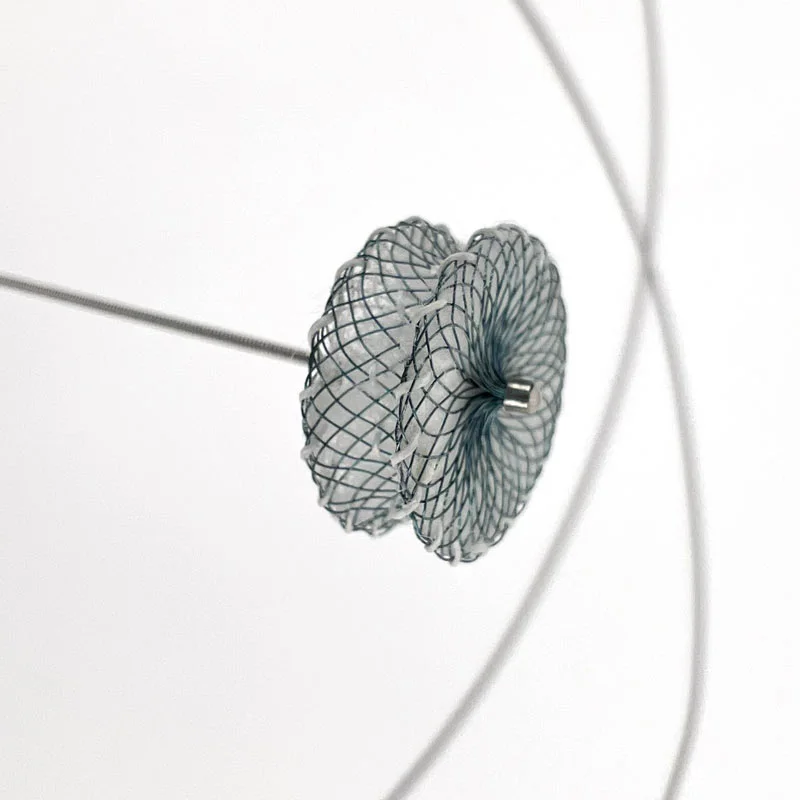
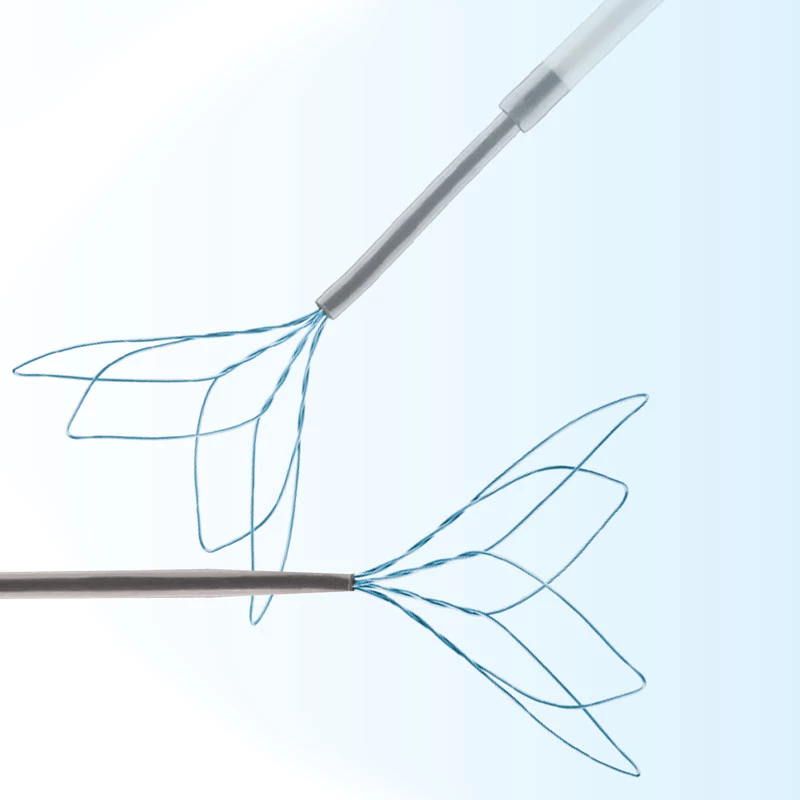
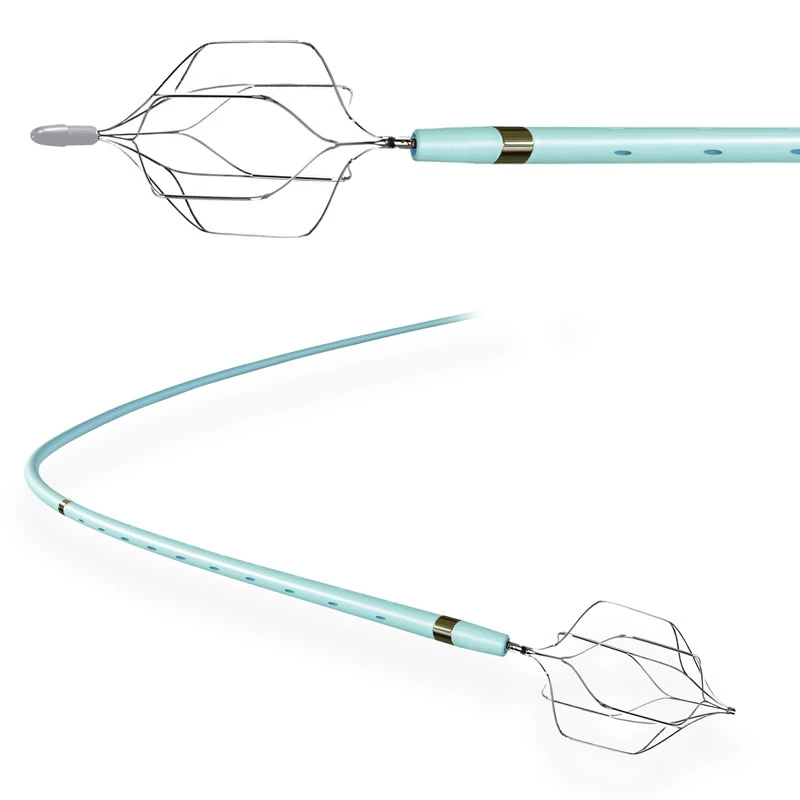
Microcatheters are also available with two radiopaque markers, 2RO, designed to assist in the delivery of detachable embolic coils during embolization procedures.
A SAFE EMBOLOTHERAPY
- Coil design reinforcement for bending resistance
- PTFE inner layer designed for exceptional guidewire tracking with virtually frictionless conduction of coils and other embolic
- DMSO Compatible
- Based on mechanical testing
SUPERIOR DESIGN TO INCREASED ACCESS TO SMALL ENVIRONMENTAL VESSELS
- The hydrophilic coating improves navigation in curved anatomy
- Unique variable coil pitch construction provides distal flexibility and proximal pushability, increasing vessel selectivity and catheter placement
FAST, PRECISE POSITIONING WITH OPTIMAL VISIBILITY
- Radiopaque platinum/iridium microcatheter marker
A Safe Embolotherapy
- Coil design reinforcement for bending resistance.
- PTFE inner layer designed for exceptional guidewire tracking with virtually frictionless conduction of coils and other embolic.
- DMSO Compatible.
- Based on mechanical testing.
| Order Number | Device inner diameter | Working Length | Design |
| VS1155 | 0.5 mm | 150 cm | Braided, Marked |
| VS1156 | 0.5 mm | 90 cm | Braided, Double Marked |
| VS1157 | 0.6 mm | 150 cm | Marked |
| VS1158 | 0.6 mm | 90 cm | Double Marked |
| VS1159 | 0.7 mm | 150 cm | Braided, Marked |
| VS1160 | 0.7 mm | 90 cm | Braided, Double Marked |
| VS1161 | 0.7 mm | 150 cm | Double Marked |
| VS1162 | 0.7 mm | 90 cm | Double Marked |